Some of these safety devices include blunt suture needles, needle less devices, shielded needles and the use of plastic tubes. When these devices are shown to effectively reduce or eliminate the hazard, they must be used.
Below is a list of design features relating to needle systems the FDA has identified and OSHA feels are important to reducing hazards of accidental injury:
1. In order to provide a barrier between the hands and the needle before, during and after use, a fixed safety feature should be included in the design.
2. This safety feature should be an integral part of the device and not a removable accessory.
3. The safety feature should be in effect before disassembly and should remain in effect after disposal to protect the user and those that handle the trash.
4. The safety feature should be simple, requiring little or no training for effective use.
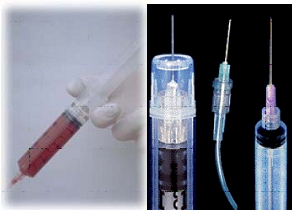
 Examples of safe needle devices:
Examples of safe needle devices:
· After the final tube of blood is drawn the needle point is blunted before it is removed from the patient or the needle point is shielded immediately after it is removed from the patient.
· A study of phlebotomy needles demonstrated an 82% reduction in needlesticks after the implementation of a blood collection tube holder that incorporated an after-use needle shield.
· Retracting or self-blunting butterfly needles
* A 25% reduction in needlesticks was reported with the use of a butterfly-type needle which incorporates a protective sliding shield, compared to a conventional butterfly-type needle
· Safety syringes where a cylindrical sheath shields needles when blood is being injected into tubes.
* For injecting blood into a tube, the sheath can be locked into place over the needle and a blood tube can be inserted into the sheath so that injection of blood occurs within the protective sheath. A 1992 trial of resheathing safety syringes resulted an 86% reduction in needlesticks compared to traditional syringes.
· Self-blunting suture needles
* Suture needles are the third highest cause of reported percutaneous injuries in US hospitals and the top cause of percutaneous injuries in the surgical setting. Compared to hollow-bore needles, however, suture needles have a much smaller potential for transferring blood from a patient to a health care worker during a needlestick.
As a general practice, bending needles, replacing caps, or removing contaminated needles is not acceptable. Shearing or breaking of needles is also prohibited unless demonstrated that no alternative is feasible or the action is required by the manufacturer or specific procedure. In this case the action must be accomplished through the use of a mechanical device or a one handed method.
Needles should be used and immediately discarded, un-recapped, into accessible sharps containers. In a few procedures, such as using the same needle with the same medication on the same patient, a needle may be recapped. But this recapping must be performed by a method other than the two-handed method.
