

All workers should take precautions to prevent injuries caused by needles, scalpel blades, and other sharp instruments. When cleaning used instruments, you should never place your hands into a container or solution you cannot see into such as a sink of soapy water or a covered container. After the sharps are used, they should be place in puncture-resistant containers located close to their use.
Broken glassware visibly contaminated with blood must be picked up with a brush and dustpan. Hands must be covered with gloves, but the hand must not touch the glass. Once the broken glass has been picked up it should be decontaminated with an approved disinfectant or steam sterilized.
Decontaminated glassware is then placed in a labeled, closeable, puncture-resistant container. Blood in the area of the broken glass must be cleaned with an approved solution, allowing the solution to remain on the blood for a period of 10 or more minutes or as recommended by the manufacturer.
 Appropriate containers for used sharps and needles must:
· Be Puncture resistant
Appropriate containers for used sharps and needles must:
· Be Puncture resistant
· Be properly labeled or color coded
· Be leakproof on the sides and the bottom
· Not require the employee to reach into the container where used sharps or needles have been placed.

A sharps container should be closeable and labeled. The container material can be any material that can be said to be leakproof on the sides and bottom, and it must also be puncture resistant. A needle sheath is never to be considered a safe container. A needle with a sheath attached must also be disposed of in a sharps container.
Overfilling sharps containers is never permitted. If the sharps container does not have a window for viewing the contents, it should be placed at a height to determine it is no more than 75% full. Sharps containers must be closed before moving, and should be sealed to prevent leakage from liquid left in the needle.
If the container cannot be sealed to prevent leakage, it must have a leak proof lining. The containers must be durable enough to contain the waste over a period of time until the waste can be incinerated or disinfected.
 Examples and Minimum Requirements for Engineering Controls:
Examples and Minimum Requirements for Engineering Controls:
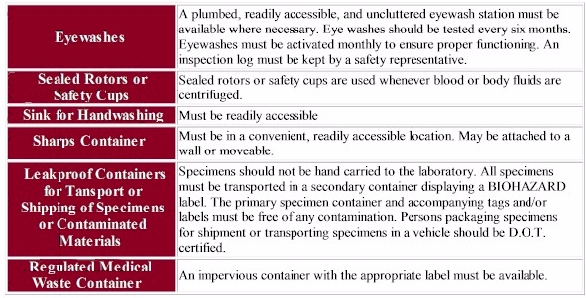
 All engineering controls must be reviewed and replaced when it is shown the device is not effectively minimizing risk. This review will also confirm that safer devices continue to function in the manner they were intended. It is important to note when safety designs are being removed or bypassed. Those controls that must be replaced regularly should be examined periodically and maintained or replaced when they no longer function as designed.
All engineering controls must be reviewed and replaced when it is shown the device is not effectively minimizing risk. This review will also confirm that safer devices continue to function in the manner they were intended. It is important to note when safety designs are being removed or bypassed. Those controls that must be replaced regularly should be examined periodically and maintained or replaced when they no longer function as designed.
Before you go on to the Quiz, check your understanding of the following concepts and vocabulary.
· Engineering practice controls
· Safety features
· FDA
· Needle designs
· Recapping
· Sharps
· Cleaning and processing
· Broken glassware
· Containers
